Product Details
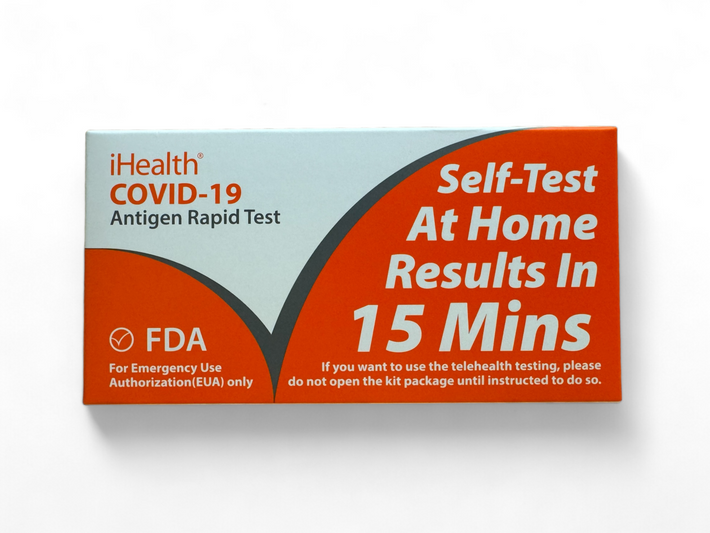
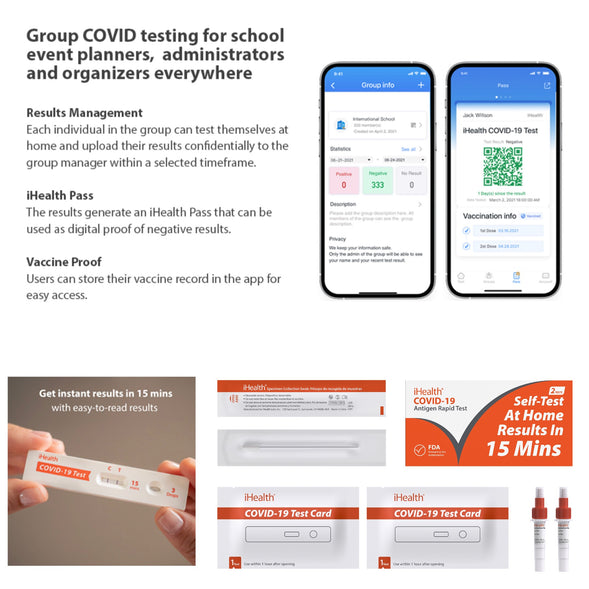
The iHealth COVID-19 Antigen Rapid Test offers a quick, non-invasive solution for detecting the SARS-CoV-2 nucleocapsid protein antigen. With just four simple steps and results in 15 minutes, this test is designed for easy home use, making it ideal for households with children as young as two years old. The test eliminates the need for deep nasal cavity sampling while still providing accurate results. iHealth has completed in-lab testing on several heat inactivated variant strains and the results show that iHealth COVID-19 Antigen Rapid Test was able to detect the mutations. Approved by the FDA under an Emergency Use Authorization (EUA), the iHealth COVID-19 Antigen Rapid Test ensures reliable, over-the-counter testing for peace of mind during uncertain times.
Manufacturer Details
iHealth COVID-19 Antigen Rapid Tests are manufactured for iHealth Labs, Inc. located in Sunnyvale, California.
Lot No. (10): 241CO20125
Expiration date: 04/24/2025
Usage Details
Instructions for use are on the product package.
Packaging Details
Case dimensions:[ 13.1” x 11.8” x 10.8”]
Case weight: Approx 12.8 lbs
Inner: Box of 2 pcs
Outer: Each case of 180 pcs contains 90 boxes x 2 pcs/box.
We make sure our products are verified and vetted